First vanadium pentoxide from QLD industrial waste: QEM and UQ collab
QEM (ASX: QEM) and The University of Queensland (UQ) have teamed up to produce the first high-purity vanadium pentoxide (V2O5) from an industrial waste stream in Australia.
In June last year, QEM announced an agreement with Incitec Pivot Limited (ASX: IPL) to collect vanadium-bearing spent catalyst from IPL’s Mount Isa sulphuric acid plant and process this waste into high-purity vanadium pentoxide (V2O5), an essential component of the electrolyte used in vanadium flow batteries.
QEM also entered a similar agreement with Sun Metals Corporation Pty Ltd’s Townsville zinc refinery in March last year.
Currently the spent catalyst removed from sulphuric acid plants ends up in waste facilities.
Test work previously conducted by QEM and Clean-Teq Water, established that 90% of the battery grade vanadium present can be extracted using known techniques.
The collaboration with UQ is a further step in demonstrating vanadium recovery.
This research and development project is led by UQ Associate Professor James Vaughan and is part of the Resources Technology and Critical Minerals Trailblazer which aims to advance critical mineral processing technology readiness.
“This project is an exciting demonstration of the circular economy of vanadium, the key ingredient in vanadium flow batteries which can provide large-scale and long-duration energy storage, complementing renewable electricity generation,” he said.
The first stage of the collaboration between QEM and UQ involved a small-scale laboratory demonstration of all processing steps in recycling the spent catalyst into a high purity vanadium oxide product.
QEM managing director Gavin Loyden says “the waste recycling project with UQ ties strongly to our ESG goals to position QEM’s projects and activities at the forefront of environmental and social responsibility within the mining and energy sectors”.
“The collaboration with UQ builds on QEM’s umbrella agreement with the university from September 2022 when The University of Queensland Sustainable Minerals Institute (UQ SMI) commenced mineral characterisation and beneficiation work for QEM’s flagship critical minerals project by characterising the mineralogy of QEM’s Julia Creek shale post-oil extraction to assist in optimising vanadium beneficiation to further improve vanadium pentoxide yields.
“With UQ’s assistance, QEM seeks to accelerate the introduction of Queensland-sourced and processed V2O5 into the market.
“QEM remains committed to its goal of supplying V2O5 from our primary vanadium resource at Julia Creek.”
SMI director Professor Rick Valenta, who also leads the UQ Trailblazer Universities Program, says “the SMI is committed to supporting sustainable extraction of vanadium from the Julia Creek region, and in that context our partnership with QEM has been both strategic and important”.
“This recycling project is an important part of this overall program.
“We are looking forward to continuing to work together to help develop these resources and contribute to the development of Queensland’s critical minerals industry.
“This is exactly the kind of project the Trailblazer Universities Program was designed to promote and as an excellent example of universities, researchers and industry working together.”
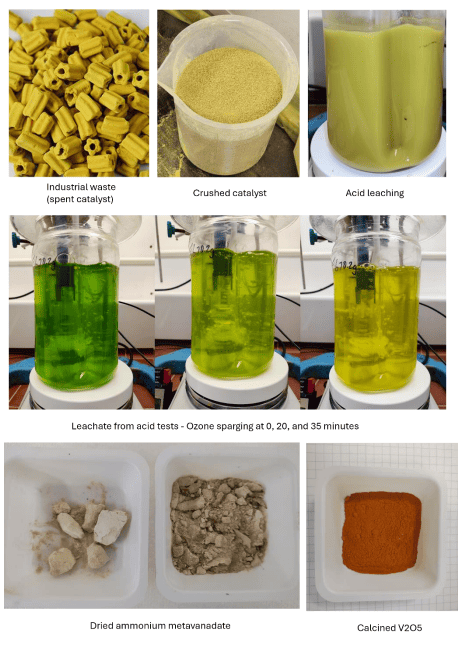
The work undertaken by UQ included laboratory test work to determine the required process conditions for an acid leach/solvent extraction flowsheet for the spent catalyst.
The program also included assaying the V2O5 that was produced in the study to determine purity of the product.
Acid leaching tests were conducted with various solid loading concentrations, acid concentrations and different feed grind sizes.
After filtration of the slurry, oxidation of the leachate and partial neutralisation were performed; then, solvent extraction was utilised to reject impurities and provide a more concentrated feed for ammonium metavanadate (AMV) crystallisation.
Finally, calcining of the AMV was performed to obtain V2O5.







































